CARCINOEMBRYONIC ANTIGEN - CAE:
Why CAE Tumor Marker Test?
CLINICAL INFORMATION
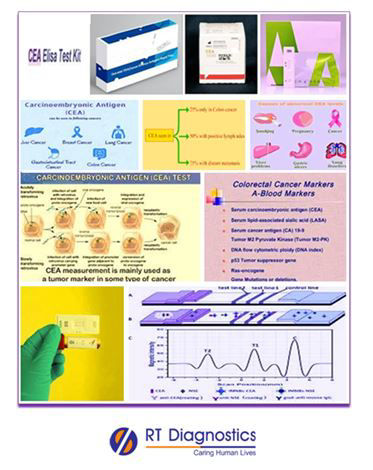
Carcinoembryonic Antigen (CAE) is known as carcino-embryonic antigen - a protein found in tissues of developing fetus and these levels gradually decrease after childbirth and are absent during adulthood. Healthy adults should have very low or absence of CEA levels in their bodies. CEA is a non-specific serological test performed to monitor certain malignant tumors especially adeno-carcinomas (adenocarcinomas are carcinomas that begin in a gland or an organ – often in the mucus membrane, thus any cancer classified as adenocarcinoma can produce high CEA levels in the body ) and is also found to be increased under non-malignant conditions like stress, aging, smoking, insulin resistance, low-grade inflammation, liver cirrhosis (scarring of the liver), emphysema, non-cancerous breast diseases, pancreatitis, inflammatory bowel diseases, diverticulitis, gall bladder inflammation, rectal polyps, inflammation of gall bladder, etc. A tumor marker is a substance produced by the cancer cells or substances that can be produced by non-cancerous (adjacent) cells in response to any cancer or benign (non-cancerous) conditions and are released into the bloodstream, which provides information about cancer. CEA tumor marker is a screening test performed in the blood or other body fluids (CSF, peritoneal fluid, pleural fluid) that helps to check cancers (rectum, thyroid, lung, breast, prostate, liver, cancers of the pancreas, tumors of the GIT eg. stomach, cancers of the urinary or reproductive tracts, head & neck cancers and ovarian cancers) mainly medullary thyroid cancers, cancers of the colon – i.e colo-rectal cancers, cancer recurrence and also to monitor the response to treatment i.e chemotherapy, radiation therapy, resection or excision surgeries, etc (rate of cancer regression after surgical treatment). Hence this CAE test can assist in early diagnosis and thus detects early symptoms of carcinogenesis, but its confirmation is mandatory along with other additional tests (evidence) along with other supporting clinical investigations, which remains as the mainstay in diagnostic confirmation of cancer associated with CEA tumor marker antigen. CEA levels can also be increased in certain non-cancerous conditions such as stress (stress due to immobilized conditions and patients who were confirmed with certain cancers like chronic lymphocytic leukemia – CLL, felt more stressed and anxious about their conditions had higher levels of tumor marker levels and increased volumes of cancer cells in the body), are found to elevated CEA levels and moreover increased levels of stress is directly found to elevate tumor marker expression and also in increased tumor growth. Therefore CEA test being a non-specific indicator is not used as such for cancer diagnosis, because not all cancers cause CEA levels to rise (only adeno-carcinomas) on the contrary certain non-cancerous conditions can cause CEA levels to increase, but nevertheless, if a patient has already been diagnosed with cancer, CEA test can help monitor the spread of cancer to different parts of the body (metastasized) and also the effectiveness of the treatment (prognosis). Other tests include additional relevant tumor marker studies, clinical investigations – signs and symptoms, ultrasound studies, CT and MRI scan (imaging studies), histo-pathological studies, etc.
General Instructions:
Sample Requirement: Specimen - Blood sample collected from the vein. Test Preparation: None.
NOTE - Sample for specimen collections may vary based on the patient’s condition/cases according to the patient’s presenting complaints/signs or symptoms:
SPECIMEN REQUIREMENT (Special or Rare Cases) - As instructed and guided by Physician / Clinician / Pathologist / as per Laboratory’s requirements, according to procedures and protocols.
Sample Requirement: Blood Sample taken from the vein
Test Preparation: None
This Multi-Specialty Clinical Referral Laboratory RT DIAGNOSTICS provides precise and accurate tests with an extensive range of testing services to the medical centers to help in the diagnosis and identification of pathology in the test specimens for infectious diseases and also to evaluate the function of organ systems of the patient. It prevents further complications and helps to stabilize and restore health to near normalcy at the earliest without delay.



